Rational surface engineering of an arginine deiminase (an anti‐tumor enzyme) for increased PEGylation efficiency
用于提高PEG化效率的精氨酸脱亚胺酶(抗肿瘤酶)有理曲面工程化
Abstract
摘要
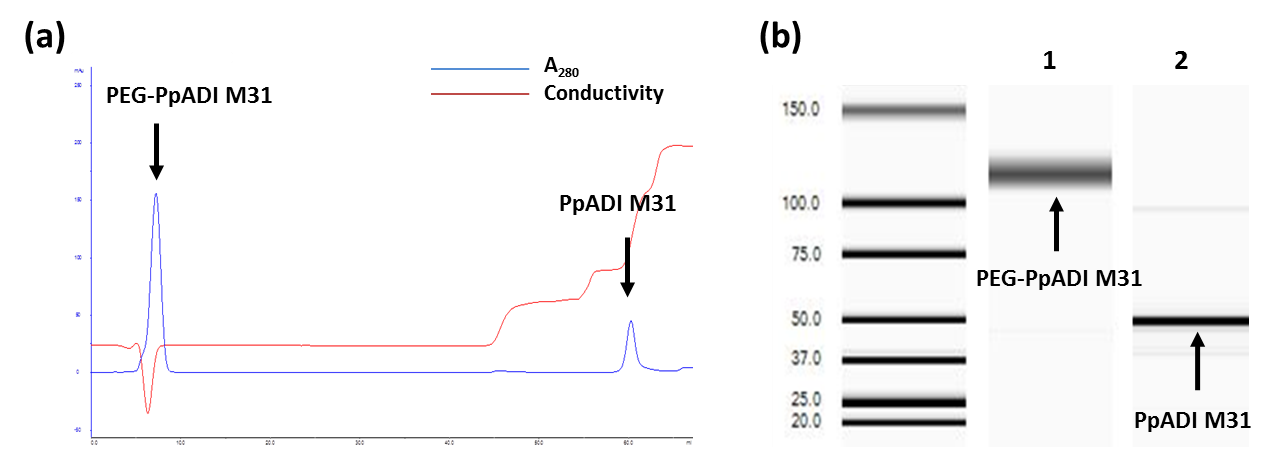
Arginine deiminase (ADI) is a therapeutic protein for cancer therapy of arginine‐auxotrophic tumors. However, its application as anti‐cancer drug is hampered by its poor stability under physiological conditions in blood stream. Commonly, random PEGylation is being used for increasing the stability of ADI and in turn the improved half‐life. However, the traditional random PEGylation usually leads to poor PEGylation efficiency due to the limited number of Lys on the protein surface. In order to boost the PEGylation efficiency and enhance the stability of ADI further, surface engineering of PpADI (an ADI from Pseudomonas plecoglossicida) to increase the suitable PEGylation sites was carried out. A new in silico approach for increasing the PEGylation sites was developed. The validation of this approach was performed on previously identified PpADI variant M31 to increase potential PEGylation sites. Four Arg residues on the surface of PpADI M31 were selected through three criteria and subsequently substituted to Lys, aiming for providing primary amines for PEGylation. Two out of the four substitutions (R299K and R382K) enhanced the stability of PEGylated PpADI in human serum. The average numbers of PEGylation sites were increased from ~12 (tetrameric PpADI M31, starting point) to ~20 (tetrameric PpADI M36, final variant). Importantly, the PEGylated PpADI M36 after PEGylation exhibited significantly improved T m values (M31: 40 °C; M36: 40 °C; PEG‐M31: 54 °C; PEG‐M36: 64 °C) and half‐life in human serum (M31: 1.9 days; M36: 2.0 days; PEG‐M31: 3.2 days; PEG‐M36: 4.8 days). These proved that the surface engineering is an effective approach to increase the PEGylation efficiency which therefore enhances the stability of therapeutic enzymes. Furthermore, the PEGylated PpADI M36 represents a highly attractive candidate for the treatment of arginine‐auxotrophic tumors.
精氨酸脱亚胺酶(ADI)是用于精氨酸营养缺陷型肿瘤的癌症治疗的治疗性蛋白质。然而,其作为抗癌药物的应用受到其在生理条件下血流中稳定性差的阻碍。通常,随机PEG化用于增加ADI的稳定性,进而改善半衰期。然而,由于蛋白质表面上Lys的数量有限,传统的随机PEG化通常导致差的PEG化效率。为了进一步提高PEG化效率和增强ADI的稳定性,进行PpADI(一种来自假单胞菌的ADI)的表面工程化以增加合适的PEG化位点。一种用于增加PEG化位点的新的计算机方法被开发了。对先前鉴定的PpADI变体M31进行该方法的验证以增加潜在的PEG化位点。通过三个标准选择PpADI M31表面上的4个Arg残基,随后用Lys替换,为聚乙二醇化提供伯胺。四种取代中的两种(R299K和R382K)增强了PEG化PpADI在人血清中的稳定性。PEG化位点的平均数量从~12(四聚体PpADI M31,起始点)增加至~20(四聚体PpADI M36,最终变体)。重要的是,PEG化后聚乙二醇化的PpADI M36表现出显着改善的T m值(M31:40℃; M36:40℃; PEG-M31:54℃; PEG-M36:64℃)和人血清中的半衰期(M31:1.9天; M36:2.0天; PEG-M31:3.2天; PEG-M36:4.8天)。 这些证明表面工程是提高PEG化效率的有效方法,因此增强了治疗酶的稳定性。 此外,PEG化的PpADI M36代表了用于治疗精氨酸营养缺陷型肿瘤的极具吸引力的候选物。
首次发表: 2019-5-7 https://doi.org/10.1002/bit.27011