Abstract
NADPH oxidase 5 (NOX5) is a transmembrane signaling enzyme that produces superoxide in response to elevated cytosolic calcium. In addition to its association with numerous human diseases, NOX5 has recently been discovered to play crucial roles in the immune response and cardiovascular system. Details of NOX5 maturation, and specifically its response to changes in intracellular heme levels have remained unclear. Here we establish an experimental system in mammalian cells that allows us to probe the influence of heme availability on ROS production by NOX5. We identified a mode of dynamic regulatory control over NOX5 activity through modulation of its heme saturation and oligomeric state by intracellular heme levels and Hsp90 binding. This regulatory mechanism allows for fine-tuning and reversible modulation of NOX5 activity in response to stimuli.
Keywords
1. Introduction
The biological role of NADPH oxidase (NOX) enzymes (EC 1.6.3) is to generate reactive oxygen species (ROS) such as superoxide and H2O2 in a controlled fashion for cellular processes. ROS production must be tightly regulated because elevated levels are implicated in aging and human disease [[1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16]], while lower than optimal levels can disrupt cell signaling [7,10], proliferation [15,17], vasodilation [15,18], protein phosphorylation [13,19], and host defense [[20], [21], [22]].
The mammalian NOX family is comprised of seven members: NOX1-5, DUOX1 and DUOX2. NOX enzymes share a number of conserved features including six transmembrane domains and cytosolic N and C termini, with C-terminal NADPH and FAD binding sites. Four highly conserved heme ligating histidine residues bind two heme groups within the membrane [10]. NOX enzymes transfer NADPH electrons through FAD to the membrane-bound hemes and finally to oxygen, producing superoxide or H2O2 [23,24].
NOX5 is a Ca2+-responsive NADPH oxidase implicated in a number of human diseases, such as cancer [25,26], diabetes [14,27], and cardiovascular disorders [[28], [29], [30], [31], [32], [33], [34]]. More recent work has begun to uncover NOX5’s physiological roles in human health. NOX5 was identified as a regulator of vascular contraction, linking Ca2+ and redox signaling [35], discovered to be a key driver in the differentiation of circulating monocytes into dendritic cells [36] and found to be required for the differentiation of oligodendrocytes [37]. Dysregulation of NOX5 is associated with amplified Ca2+ signaling, vascular hypercontractility and cardiac fibrosis [35]. The peptide hormones angiotensin-II (Ang-II) and endothelin-1 (ET-1) increase NOX5 expression and activity [30,35], as do the kinases protein kinase C α (PKCα) [[38], [39], [40]], calcium and calmodulin-dependent protein kinase II (CAMKII) [41], and c-Abl kinase [42]. Despite its apparent importance in human health and disease, NOX5 is mysteriously missing in mice and rats, making it difficult to study its effects in mammals.
NOX5 activity is regulated at the post-translational level through protein phosphorylation [[38], [39], [40], [41], [42]], S-nitrosation (SNO) [43], and protein-protein interactions [44]. However, little is known about the NOX5 maturation process and the factors that control it. Although some facets of maturation of the prototypical NOX, NOX2, are known, it is likely that those details are not relevant to NOX5. NOX2 is highly glycosylated and its activity requires interactions with the membrane protein p22phox and the cytosolic factors p47phox, p67phox, p40phox and Rac [10,20]. In contrast, NOX5 is not glycosylated and its activity is not dependent upon p22phox or cytosolic factors, but is instead activated by Ca2+ binding to its EF hand domains [10,20]. Additionally, there remains the question of when, where and how heme insertion takes place in NOX5, and how changes in intracellular heme levels impact NOX5 superoxide generation. Increased intracellular heme levels have been shown to effect cells in an NAPDH oxidase dependent manner, including inflammatory responses in alveolar macrophages [45] and activation of intestinal epithelial cells [46]. As NOX5 has been found to play crucial roles in immunity and cardiovascular function, and stands as a nexus of calcium-redox signaling in the cell, understanding how NOX5 responds to changes in intracellular heme levels is crucial to understanding its regulation and role in driving cellular responses associated with changes in cellular heme content.
Recent work in our lab and others has shown that Hsp90 is directly involved in heme insertion into a number of heme proteins such as soluble guanylate cyclase (sGC) [47,48], inducible nitric oxide (NO) synthase [49], hemoglobin β and γ [50], and myoglobin [51]. Whether this reliance on Hsp90 is conserved for integral membrane heme proteins like the NOX family remains unclear. Inhibition of Hsp90 has been shown to decrease the activity of NOX5 [52], but whether this occurs through inhibition of heme insertion or through other mechanisms is unknown. Additionally, whether heme must be inserted co-translationally, or post-translationally is unresolved. We sought to understand the maturation and regulation of NOX5 in terms of heme binding, and the role of Hsp90.
To address these questions, we developed an experimental system in which cells express and accumulate heme-free (apo) NOX5. Then, we probed the effects of heme or Hsp90 inhibition on the ability of apo-NOX5 to obtain heme and regain activity. We found: (i) apo-NOX5 builds up to a significant extent in both normal and heme-depleted cells. (ii) Upon heme addition apo-NOX5 becomes fully active. (iii) Hsp90 inhibition blocks the heme-dependent stimulation of NOX5. (vi) Heme and Hsp90 regulate NOX5 activity by regulating its oligomerization status in a dynamic manner. Such multi-level post-translational regulation of NOX5 is novel and has important implications for understanding its emerging roles in physiology and disease.
2. Materials and methods
2.2. Cell culture and transient transfection
COS-7 (ATCC), HEK293 (ATCC) and HEK293 cells stably expressing NOX5 [53] were maintained in Dulbecco’s modified Eagle’s medium (DMEM) with 10% FBS and antibiotic-antimycotic (Gibco, 1X, COS-7 and HEK293 cells) or G418 (Gibco, 400 μg/mL, HEK293 cells stably expressing NOX5) at 37 °C with 5% CO2 in a humidified incubator. PC-3 cells were maintained in Kaighn’s Modification of Ham’s F-12 Media (ATCC, F–12 K) with 10% FBS and antibiotic-antimycotic. BET1A cells [54], human bronchial epithelial cells transformed with SV40, were cultured as described [55] in serum-free LHC9 media with penicillin/streptomycin on plates precoated with coating media (LHC basal, Biofluids/Biosource) containing 0.03 mg/mL collagen (Vitrogen, Cohesion Technologies), 0.01 mg/mL BSA (Biofluids/Biosource) and 0.01 mg/mL fibronectin (Cal Biochem). For transfection, COS-7 or HEK293 cells were grown in DMEM with 10% FBS and no antibiotic. Cells were transfected with 5 μg DNA and 15 μL Lipofectamine 2000 (ThermoFisher Scientific) per 10 cm dish. Sixteen hours after transfection the media was changed to DMEM with 10% FBS and 1X antibiotic-antimycotic. For heme-depleted conditions, the media was made with heme-depleted FBS. Heme-depleted FBS was made by treating serum with 10 mM ascorbic acid until absorbance at 405 nm had decreased by half, followed by dialysis against PBS and sterile filtration [56]. Cells grown in this heme-depleted (HD) media were then treated with 400 μM succinyl acetone (Sigma-Aldrich) for three days to inhibit heme synthesis and deplete cellular heme reserves.
2.3. Western blot analysis
Cell lysates were normalized to total protein content as determined by a DC protein assay (Bio-Rad). SDS-PAGE and Western blot analysis were performed using standard procedures with antibodies against NOX5 (Proteintech), Hsp90 (Abcam) and β-actin (Sigma-Aldrich). Horseradish peroxidase-linked secondary antibodies (anti-rabbit: Cell Signaling Technologies, anti-mouse: Abcam) were used to visualize the proteins using a Western Lightning Plus-ECL kit from PerkinElmer Life Sciences. Blots were imaged on a Bio-Rad ChemiDoc Imaging System and analyzed using Bio-Rad Image Lab software v5.2.1.
2.4. Labile heme measurements using a fluorescent heme sensor
HEK293 cells and heme-depleted HEK293 cells were transfected with pcDNA3.1-HS1 [57] heme sensor as described above. At 16 h post-transfection, media was replaced with corresponding media and treated with 5 μM hemin for 2 h. Cells were removed from the dish, washed 1X with 1X PBS, and re-suspended in 1X PBS + glucose. 100,000 mKATE2 positive cells were analyzed on a Becton-Dickinson LSRFortessa for mKATE2 (excitation (ex.) = 588 nm, emission (em.) = 620 nm) and GFP (ex. = 488 nm, em. = 510 nm). Data was analyzed using FlowJo v10.
2.5. UV–visible spectroscopy with Olis Clarity
The Olis Clarity is a specialized spectrophotometer that takes absorbance measurements in the UV–visible range in turbid solutions, allowing for measurements on intact cells under physiological conditions. Cells were grown in phenol red free DMEM/F-12 media with 15 mM HEPES and l-glutamine with 10% FBS and 400 μg/mL G418 (or antibiotic-antimycotic, 1X, for HEK293 control cells). Cellular heme-depletion was carried out as described above. Cycloheximide (10 μg/mL) was added to the cell culture media and cells were incubated with hemin (5 μM, 2 h), and/or radicicol (40 μM, 2 h). After incubation each sample was normalized to 1 × 107 cells in 1 mL phenol red-free media. Using the phenol red free media as a reference the absorbance (350–650 nm) of the live cell samples were read before and after addition of dithionite. Reduced minus oxidized spectra were plotted using GraphPad Prism v7.
2.6. Superoxide measurements
Cells were grown in phenol red free DMEM/F-12 media with 15 mM HEPES and l-glutamine with 10% FBS and 400 μg/mL G418 (or antibiotic-antimycotic, 1X, for HEK293 control cells). Cellular heme-depletion was carried out as described above. After treatment with cycloheximide, cells were re-plated into clear bottom white tissue culture-treated 96-well plates (Costar) at a density of 5 × 104 cells/well. Radicicol and hemin were added at various timepoints and 400 μM L-012 (Wako Chemicals) was added 10 min prior to read. The NOX5 superoxide burst was initiated by automated addition of 1 μM ionomycin and luminescence was recorded every 10 s for 300 s on a FlexStation 3 microplate reader (Molecular Devices). Superoxide levels were also measured via the cytochrome c reduction assay. Cells were treated as described, but instead of L-012, 100 μM cytochrome c was added to the wells and absorbance at 550 nm was measured using a SpectraMax M2e plate reader (Molecular Devices). Signal from HEK293 cells that do not express NOX5 were subtracted from the signal from cells that stably express NOX5 and the rate of cytochrome c reduction (nmoles/min/106 cells) was calculated using the extinction coefficient for reduced cytochrome c of 21.1 mM-1 cm-1 [58]. Superoxide levels were also measured using coelenterazine luminescence. Cells were treated as described, but instead of L-012, 50 μM coelenterazine was added to each well 10 min prior to read. Luminescence was measured as for L-012 and the signal from HEK293 cells that do not express NOX5 were subtracted from the signal from cells that stably express NOX5 for each treatment condition. Data were plotted and analyzed using GraphPad Prism v7.
2.7. Co-immunoprecipitation
Co-immunoprecipitation was carried out using standards procedures. Cell lysates normalized to total protein content were incubated with anti-NOX5 antibody (Proteintech) followed by Protein A Sepharose 4B beads overnight at 4 °C with gentle rotation. After washing the precipitated protein was subjected to SDS-page and western blot analysis as described above.
2.8. Proximity ligation assay with Duolink
Proximity Ligation Assays (PLA, Duolink, Sigma) for co-localization were performed according to the manufacturer’s protocol using primary antibodies to Hsp90 (Abcam, 1:200) and NOX5 (Proteintech, 1:1000), followed by a pair of oligonucleotide-labeled secondary antibodies (included in Duolink Kit). The assay detects positive signal only when the epitopes of the target proteins are in close proximity (<40 nm). The signal from each of the detected pair of PLA probes was then imaged using fluorescence microscopy (excitation/emission for Duolink red: 594/624; excitation/emission for Dapi: 360/460). One or both primary antibodies were omitted for negative controls.
2.9. NOX5 oligomerization assays
HEK293 cells stably expressing NOX5 were incubated with hemin (5 μM, 2 h) or radicicol (40 μM, 2 h), then treated with 1 μM ionomycin or buffer control and immediately lysed. Lysates were prepared in sample buffer without reducing agent and heated at 65 °C for 2 min. Western blotting was performed as described above. Quantification was carried out using Image Lab v5.2.1. The total density of the high molecular weight oligomers was divided by the density of the monomer band. Each treatment condition was then normalized to the untreated oligomer/monomer ratio for either the no ionomycin or 1 μM ionomycin sample sets.
2.10. Quantification and statistical analysis
Statistical parameters including the exact value of n, the statistical test used to analyze the specific data set (two-tailed Student’s t-test or one-way anova with appropriate post-test), and the parameters for statistical significance are reported in the Figures and Figure Legends. Data is presented as means ± SEM, and n represents biological replicates. Significance is denoted in the Figures with asterisks, and p-value cut-offs for the various statistical tests are listed in the Figure Legends (* denoting p < 0.05, **p < 0.01 and ***p < 0.001, for one-way anova, and *p ≤ 0.05, **p < 0.01, ***p < 0.0001 for two-tailed t-test). Statistical analyses were performed with GraphPad Prism 7.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit polyclonal anti-NOX5 | Proteintech | Cat#25350-1-AP, RRID:AB_2811208 |
| Mouse monoclonal anti-β-Actin | Sigma-Aldrich | Cat# A5441, RRID:AB_476744 |
| Mouse monoclonal anti-Hsp90 | Abcam | Cat# ab13492, RRID:AB_300396 |
| Goat anti-Rabbit IgG, HRP linked antibody | Cell Signaling Technology | Cat# 7074, RRID:AB_2099233 |
| Goat anti-Mouse IgG HRP linked antibody | Bio-Rad | Cat# 170–6516, RRID:AB_11125547 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Western Lightning Plus-ECL kit | Perkin Elmer Life Science | NEL104001EA |
| L-012 | Wako Chemicals | Cat#120–04891, CAS:143,556-24-5 |
| Coelenterazine | GLPBIO | Cat#GC17680, CAS:55,779-48-1 |
| 4,6-Dioxoheptanoic acid (Succinyl acetone) | Sigma-Aldrich | Cat#D1415, CAS:51,568-18-4 |
| Hemin Chloride | Sigma-Aldrich | Cat#2250, CAS:16,009-13-5 |
| Cycloheximide | Sigma-Aldrich | Cat#C4859, CAS:66-81-9 |
| Radicicol | Sigma-Aldrich | Cat#R2145, CAS:12,772-57-5 |
| Sodium hydrosulfite | Sigma-Aldrich | Cat#157953, CAS:7775-14-6 |
| Ionomycin | Sigma-Aldrich | Cat#I0634, CAS:56,092-82-1 |
| Cytochrome C | Sigma-Aldrich | Cat#C2037, CAS:9007-43-6 |
| Antibiotic-antimycotic | Gibco | Cat#15240062, CAS:3810-74-0, 69-57-8 |
| Geneticin (G418 sulfate) | Gibco | Cat#10131035, CAS:108,321-42-2 |
| Vitrogen 100 | Cohesion Technologies | Cat#FXP-019 |
| BSA 10X | Biofluids/Biosource | Cat#343-020 |
| Fibronectin 5 mg/mL | Cal Biochem | Cat#341631 |
| LHC Basal | Biofluids/Biosource | Cat#P118-500 |
| Critical Commercial Assays | ||
| Duolink In Situ Red Starter Kit Mouse/Rabbit | Sigma-Aldrich | DUO92101 |
| DC Protein Assay Kit II | Bio-Rad | 5000112 |
| Experimental Models: Cell Lines | ||
| COS-7 | ATCC | ATCC#CRL-1651, RRID:CVCL_0224 |
| HEK293 | ATCC | ATCC#CRL-1573, RRID:CVCL_0045 |
| HEK293, hNOX5, clone B2 | Banfi et al., 2001 | N/A |
| PC-3 | ATCC | ATCC#CRL-1435, RRID:CVCL_0035 |
| BET1A | Reddel et al., 1988 | RRID:CVCL_0171 |
| Recombinant DNA | ||
| pcDNA3.1-hNOX5 | Banfi et al., 2001 | Addgene: 69,354 |
| pcDNA3.1-hNOX5 H268G | This study | N/A |
| pcDNA3.1-hNOX5 H268A | This study | N/A |
| pcDNA3.1-hNOX5 H268L | This study | N/A |
| pcDNA3.1-hNOX5 H282G | This study | N/A |
| pcDNA3.1-hNOX5 H282A | This study | N/A |
| pcDNA3.1-hNOX5 H282L | This study | N/A |
| pcDNA3.1-hNOX5 H286G | This study | N/A |
| pcDNA3.1-hNOX5 H286A | This study | N/A |
| pcDNA3.1-hNOX5 H286L | This study | N/A |
| pcDNA3.1-hNOX5 H356G | This study | N/A |
| pcDNA3.1-hNOX5 H356A | This study | N/A |
| pcDNA3.1-hNOX5 H356L | This study | N/A |
| pcDNA3.1-hNOX5 H369G | This study | N/A |
| pcDNA3.1-hNOX5 H369A | This study | N/A |
| pcDNA3.1-hNOX5 H369L | This study | N/A |
| pcDNA3.1-hNOX5 P420G | This study | N/A |
| pcDNA3.1-hNOX5 P549H | This study | N/A |
| pcDNA3.1-hNOX5 D638A | This study | N/A |
| pcDNA3.1-HS1 | Hanna et al., 2016 | N/A |
| Software and Algorithms | ||
| Image Lab | Bio-Rad | v5.2.1, RRID:SCR_014210 |
| Prism | GraphPad | v7, RRID:SCR_002798 |
| FlowJo | FlowJo | v10.2, RRID:SCR_008520 |
| Other | ||
| DMEM, high glucose, pyruvate | Sigma-Aldrich | Cat#D7777 |
| DMEM/F-12 15 mM HEPES, l-glutamine w/o phenol red | Sigma-Aldrich | Cat#D2906 |
| Lipofectamine 2000 | ThermoFisher Scientific | Cat#11668019 |
| LHC9 | Gibco | Cat#12680-013 |
3. Results
Apo-NOX5 persists in heme-depleted cells and remains capable of binding heme. To study heme insertion during maturation of NOX5 we adapted a protocol that we have used to study heme insertion and subsequent activity of a number of soluble heme proteins [[47], [48], [49], [50]]. HEK cells that stably express NOX5β were grown in heme-depleted media with succinyl acetone (SA) for three days to deplete intracellular heme reserves, as confirmed with an in vivo heme sensor HS1 [57] (Fig. 1a). Protein synthesis was then halted using cycloheximide, the heme-depleted cells were incubated with hemin for 2 h and the activity and heme content of NOX5 was assessed. The heme depletion conditions partially decreased cellular NOX5 expression by approximately 35% relative to cells cultured in normal media (Fig. 1b, Supp. Fig. 1a and b). To determine the heme bound status of the NOX5 we obtained reduced minus oxidized spectra on the live cells using an Olis Clarity UV–vis spectrometer (Fig. 1c–e), which allows UV–vis measurements to be taken in turbid solutions such as live cell suspensions. In cells grown in normal media, the difference spectrum displayed NOX-specific heme peaks at 429 and 558 nm [59] (Fig. 1c). These peaks were nearly abolished in the heme-depleted cells (Fig. 1d), however, addition of 5 μM hemin to these cells restored the NOX5 peaks (Fig. 1e) without affecting the NOX5 protein levels (Fig. 1f, Supp. Fig. 1c and d). HEK cells which did not express NOX5 did not display NOX5 specific heme peaks in any condition (Fig. 1c, Supp. Fig.1e). Thus, in the heme-depleted cells the majority of NOX5 was in a heme-free apo-form that retained the ability to bind provided heme, suggesting that it reflects an on-pathway step in NOX5 maturation. This allowed us to probe aspects of NOX5 maturation and heme binding.

Fig. 1. Apo-NOX5 persists under heme-depleted conditions and is able to bind exogenous heme. A) Cellular heme conditions probed using a genetically encoded heme sensor HS1 [57] and flow cytometry. The HS1 sensor contains a heme binding moiety, an eGFP sensitive to heme binding, and an mKATE2 that is insensitive to heme binding. The ratio of eGFP:mKATE2 fluorescence is indicative of labile heme content of the cell, with the ratio inversely correlated with heme concentration. The flow cytometry data are representative of HEK293 cells cultured in normal media (black), heme-depleted media treated with succinyl acetone (SA) to inhibit heme synthesis (gray) and heme-depleted media with SA and 5 μM hemin added for 2 h (red). B) Representative western blot analysis of HEK293 cells and HEK293 cells stably expressing NOX5β grown in either normal media or heme-depleted media with SA and quantification of expression in these conditions. Blots correspond to panels C and D. Quantification values represent means ± SEM, n = 6, *** denotes p < 0.001, two-tailed t-test. C-E) Reduced minus oxidized difference spectra of live cells using the Olis Clarity. After addition of cycloheximide to halt protein synthesis, cells were normalized to live cell number and the UV–vis spectrum was taken before and after addition of sodium dithionite. The oxidized spectrum was then subtracted from the reduced spectrum. C) Difference spectra of HEK293 cells (green) and HEK293 cells stably expressing NOX5β (blue). Characteristic NOX5 peaks are visible at 429 nm and 558 nm. D) Difference spectra of HEK293 cells stably expressing NOX5β grown in normal media (blue) or heme-depleted media with SA treatment (gray). NOX5 peaks are diminished in heme-depleted conditions. E) Difference spectra of HEK293 cells stably expressing NOX5β grown in heme-depleted media with SA treatment with (red) and without (gray) a 2 h incubation with 5 μM heme. Heme addition increases the NOX5 specific peaks. F) Western blot analysis of cell lysates of the samples shown in E. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Exogenous heme reconstitutes NOX5 activity. While the difference spectra suggest that adding heme to heme-depleted cells was able to reconstitute holo-NOX5 from the apo-form, it was unclear whether the resulting enzyme was active. Ca2+ binding to the N-terminal EF hands of NOX5 triggers electron transfer through the two hemes bound in its transmembrane domain, which then reduces dioxygen to superoxide. A superoxide burst by NOX5 can thus be stimulated in cells by the addition of ionomycin Ca2+ salt and measured using the superoxide sensor L-012 [43,60], or alternatively, by the cytochrome c reduction assay [52,53] or luminescence of coelenterazine (2-(4-hydroxybenzyl)-6-(4-hydroxyphenyl)-8-benzyl-3,7-dihydroimidazo[1,2-α]pyrazin-3-one) [61,62]. We measured the Ca2+-sensitive superoxide burst in cells grown in normal media, in heme-depleted conditions, and in heme-depleted conditions after a 2-h incubation with 5 μM hemin. Protein translation was inhibited with cycloheximide before any cell treatment to eliminate contributions by newly synthesized NOX5. As shown in Fig. 2a and b, superoxide production by NOX5 in heme-depleted cells was diminished by >70% compared to the activity in cells grown in normal media. The addition of 5 μM hemin restored superoxide production to normal levels (Fig. 2a and b). This effect was also time sensitive (Fig. 2c and d), consistent with a binding event such as heme insertion. Control HEK cells that do not express NOX5 did not produce measurable superoxide under any condition (Supp. Fig. 2a and b). These results confirm that apo-NOX5 can bind exogenous heme and this restores it to an active state in live cells.

Fig. 2. Heme addition restores superoxide production by NOX5 in cells grown in heme-depleted conditions, and increases superoxide production by cells grown in normal media. Superoxide levels were detected using the luminol analog L-012, which luminescences in the presence of superoxide but not other reactive oxygen species such as hydrogen peroxide. Before any incubation with hemin the cells were treated with cycloheximide to block protein translation. Addition of ionomycin Ca2+ salt triggers a NOX5 superoxide burst. A) Timecourse of superoxide production by HEK293 cells stably expressing NOX5β grown in normal media vs. heme-depleted media with SA treatment. Addition of 5 μM hemin to cell culture for 2 h results in recovery of NOX5 superoxide production. B) Quantification of peak superoxide production by NOX5. Values represent means ± SEM, n = 10. Differences between columns assessed using a one-way anova with Tukey’s test *p ≤ 0.05, **p < 0.01. C) Timecourse of superoxide production by HEK293 cells stably expressing NOX5β grown in heme-depleted media with SA treatment incubated with 5 μM hemin for 0, 10, or 120 min. D) Quantification of peak superoxide production by NOX5 with heme incubation. Values represent means ± SEM, n = 3. Differences between columns assessed using a one-way anova with Tukey’s test **p < 0.01, ***p < 0.001. E) Peak superoxide production after incubation with hemin by HEK293 cells stably expressing NOX5β in either normal media or heme-depleted media with SA treatment. Values represent means ± SEM, n = 6–12. Within each media group (normal media and heme-depleted with SA treatment) the differences in NOX5 activity with various heme concentrations was assessed using a one-way anova with Tukey’s test with * denoting p < 0.05, **p < 0.01 and ***p < 0.001. F) Western blot analysis of cell lysates from HEK293 cells stably expressing NOX5β grown in normal media and treated with either DMSO control or 5 μM hemin for 2 h.
Surprisingly, we also found that adding hemin to cells grown in normal media conditions increased NOX5 superoxide production in a concentration-dependent manner (Fig. 2e), again without affecting NOX5 protein levels as seen by Western blot (Fig. 2f). The increase in superoxide production was attributable to NOX5, because it did not occur when hemin was given to cells that do not express NOX5 (Supp. Fig. 2a and b). Additionally, the effect of heme was not an artifact of the L-012, as cell superoxide production still increased after hemin treatment whether it was assessed by cytochrome c reduction (Supp. Fig. 3a) or by luminescence of coelenterazine (Supp. Fig. 3b). Additionally, a stimulatory effect of heme was also observed in COS-7 cells transfected with NOX5β (Supp. Fig. 3c). These results imply that cells grown under normal culture conditions contain a significant subpopulation of apo- or semi-heme-bound NOX5 that is capable of incorporating exogenous heme provided to the cells.
Endogenously produced heme increases NOX5 superoxide production and endogenously produced NOX5 is stimulated by heme addition. To assess whether this increase in NOX5 generated superoxide could be physiologically relevant, we repeated our assays with endogenously produced heme as well as determined the effect of exogenous heme on endogenously produced NOX5. Endogenous heme production occurs from glycine in a number of steps carried out within the mitochondria and cytosol. By supplementing the cell with the rate limiting intermediate δ-aminolevulinic acid (δ-ala) and ferric citrate (Fe-cit), heme biosynthesis can be upregulated, resulting in an increase in endogenously produced heme [63,64]. Adding these precursors to HEK cells that stably express NOX5β resulted a time dependent increase in NOX5 superoxide production (Fig. 3a) consistent with a gradual increase in intracellular heme levels due to stimulation of the biosynthetic pathway. Protein translation was inhibited by the use of cycloheximide, which may have decreased the amount of endogenous heme produced but also ensured that the superoxide production was not due to increases in NOX5 protein levels. These results show that NOX5 superoxide production is stimulated by increases in both exogenous and endogenously produced heme.

Fig. 3. Endogenously produced heme increases superoxide production by NOX5 and endogenously expressed NOX5 is also stimulated by heme addition. Cycloheximide was added prior to any other cell treatments. A) HEK293 cells stably expressing NOX5β were grown in normal media and treated with either vehicle control, 1 or 5 μM hemin or 1 mM δ-aminolevulinic acid and 100 μM ferric citrate (δ-Ala + Fe-cit). Superoxide production upon ionomycin Ca2+ salt addition was measured after 10 min, 2 h, or 5 h. Values represents means ± SEM, n = 8 and were normalized to untreated controls at each timepoint. For each timepoint the difference in NOX5 activity with heme or δ-Ala + Fe-cit treatment was assessed using a one-way anova with Dunnett’s test with * denoting p < 0.05, and ***p < 0.001. B) PC-3 and BET1A cells were treated with vehicle control or 5 μM hemin for 2 h. Superoxide production upon ionomycin Ca2+ salt addition was measured using L-012 luminescence. Values represents means ± SEM, n = 8, and normalized to untreated BET1A control. Differences between the cells and treatments was assessed using a one-way anova with Tukey’s test with *** denoting p < 0.001. C) Western blot analysis of PC-3 and BET1A lysates.
To ensure that the effect of heme was NOX5 dependent we tested other cell lines for NOX5 expression and NOX5 superoxide production with and without addition of 5 μM hemin (Fig. 3b and c). PC-3 (prostate carcinoma) cells did not express measurable NOX5 by western blot (Fig. 3c), and did not produce a superoxide burst with the addition of ionomycin Ca2+ salt as measured using the superoxide sensor L-012 (Fig. 3b). However, BET1A (bronchial epithelial) cells did express measurable NOX5 protein (Fig. 3c) and produced superoxide in response to ionomycin Ca2+ salt (Fig. 3b). Importantly, the BET1A cells produced significantly more superoxide after treatment with 5 μM hemin (Fig. 3b), indicating that the NOX5 dependence on heme and its effect on superoxide generation is physiologically relevant.
Superoxide production by NOX5 requires bis-his ligation of both heme groups. NADPH oxidase enzymes ligate two heme groups in their transmembrane domain through four conserved histidine residues (Fig. 4a). We wanted to test the heme binding requirements of NOX5 superoxide production and therefore substituted each of the conserved heme ligating histidines with glycine, alanine or leucine. We transfected these NOX5 variants into HEK cells and measured superoxide production in response to ionomycin Ca2+ salt with and without addition of 5 μM hemin (Fig. 4b and c). Substitution of any one of the four heme-ligating histidines (His268, His282, His356 and His369, Fig. 4a) completely abolished NOX5 superoxide production (Fig. 4b). Although not all of the His variants expressed as well as the WT enzyme, most notably at the His282 position Fig. 4c), the complete elimination of superoxide production suggests that both heme groups must be bis-his coordinated for electron transfer to occur. We also made NOX5 variants with substitutions to a nearby histidine, His286, that is not involved in heme ligation, but is proposed to be involved in oxygen binding [65]. The His286Ala and His286Leu variants displayed lower activity than the WT enzyme, but retained the response to heme (Fig. 4b), while mutation of His286 to glycine nearly abolished activity of the enzyme (Fig. 4b). These findings support a critical role for this residue in oxygen coordination, and further confirm that it is specifically heme binding to NOX5 that leads to increases in superoxide production.

Fig. 4. Mutation of any heme-ligating histidine eliminates superoxide production by NOX5. A) Cartoon representation of the transmembrane region of NOX5 from PDB 5o0t [65]. Heme binding histidines (H) are shown as blue spheres (H268, H282, H356 and H369 in NOX5β) and H286, a residue predicted to be involved in O2 binding [65] is shown in teal. B) HEK293 cells were transiently transfected with NOX5β WT and histidine variants. Each histidine was mutated to glycine (G), alanine (A) or leucine (L), and activity was assessed after incubation with DMSO control or 5 μM hemin. Peak superoxide production values were normalized to untreated WT activity and represent means ± SEM, n = 3–12, *p ≤ 0.05, ***p < 0.0001, two-tailed t-test. C) Western blot analysis of NOX5β variant expression. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Heme binding and its stimulatory effect on NOX5 activity is dynamic. We next assessed the dynamics of the heme-dependent increases in NOX5 activity. HEK cells stably expressing NOX5β were treated with cycloheximide and incubated with 0, 1, 5 or 10 μM hemin for 2 h. After incubation with hemin, the media was removed from NOX5 expressing cells and replaced with fresh media containing no exogenous heme. We then stimulated superoxide production with ionomycin Ca2+ salt every 5 min for 1 h (Fig. 5a). NOX5 activity decreased after the media change, but remained elevated by 2 to 3x in the 5 or 10 μM hemin treated cells compared to untreated control. To test that the decrease in superoxide production was associated with a decrease in NOX5 bound heme, we acquired difference spectra of cells expressing NOX5β grown in normal media (Fig. 5b, gray line), after incubation with 5 μM hemin for 2 h (Fig. 5b, red line) and 35 min after fresh media was added to the heme-incubated cells (Fig. 5b, pink line). The intensity of the cells NOX5-specific heme peak decreased after the media change (Fig. 5b). Importantly, the intensity changes in the heme peak corresponded well to the NOX5 activity; the heme peak increase seen with 5 μM hemin incubation correlated with superoxide production reaching ~500% baseline (Fig. 5a and b), and 35 min after media replacement, the heme peak decreased by ~50% and the activity decreased to ~250% (Fig. 5a and b). NOX5 protein levels were not affected by hemin addition or removal (Fig. 5c). Thus, a direct correlation exists between NOX5 activity and its bound heme, with activity changing dynamically in response to changes in cellular heme levels.

Fig. 5. Heme binding and its effect on NOX5 activity is dynamic. All cells were treated with cycloheximide before heme addition. A) Decay of the effect of heme on NOX5 activity over time. NOX5β expressing cells were incubated with 1, 5 or 10 μM hemin (2 h). After incubation the media was removed and replaced with fresh media containing cycloheximide but no hemin and changes in NOX5 superoxide production was measured over time. Curves represent superoxide production normalized to untreated control at each timepoint. Each point represents mean ± SEM, n = 2–6 with *** representing p < 0.0001 for both treatment (heme) and time, two-way anova. B) Reduced minus oxidized difference spectra of live cells using the Olis Clarity. After addition of cycloheximide to halt protein synthesis, cells were treated with either DMSO control or 5 μM hemin (2 h). After incubation, media was removed from a subset of the hemin treated cells and replaced. The samples were normalized to live cell number and the UV–vis spectrum was taken before and after addition of sodium dithionite. The oxidized spectrum was then subtracted from the reduced spectrum. Characteristic NOX5 peaks are visible at 429 nm and 558 nm. C) Western blot analysis of cell lysates from B), HEK293 cells stably expressing NOX5β grown in normal media and treated with either DMSO control, or 5 μM hemin with or without subsequent removal.
Hsp90 inhibition with radicicol suppresses stimulation of NOX5 superoxide production by heme. Hsp90 interacts with NOX5 through the C-terminal cytosolic domain [66]. Short exposure (30 min) to the Hsp90 inhibitor radicicol has been shown to decrease NOX5 superoxide production [52], while longer exposure (12 h) leads to proteosomal degradation of NOX5 [67]. Hsp90 has been shown to be essential for heme insertion into a number of soluble heme proteins [[47], [48], [49], [50], [51]]. To determine if Hsp90 inhibition would affect the heme-dependent increases in NOX5 superoxide production we first confirmed that the Hsp90 inhibitor radicicol inhibited superoxide production by NOX5 in a concentration dependent manner (Supp. Fig. 4a), while an inhibitor of GRP94, the endoplasmic reticular chaperone, had no effect (Supp. Fig. 4b). We then tested the effect of radicicol on the stimulatory effect of heme. We found that at all concentrations of heme, treatment with this Hsp90 inhibitor robustly suppressed the gain in NOX5 activity (Fig. 6a) without affecting NOX5 protein levels (Fig. 6b). The inhibitory effect of radicicol on heme-induced increases in NOX5 superoxide production was also observed in COS-7 cells transfected to express NOX5β (Supp. Fig. 4c).

Fig. 6. Heme and radicicol have opposing effects on NOX5 activity and oligomerization. A) Cycloheximide was added to cells before hemin and/or radicicol treatment. Peak superoxide production by HEK293 cells stably expressing NOX5β in either normal media or heme-depleted media with SA treatment after incubation with 0, 1, 5 or 10 μM hemin with or without 40 μM radicicol. Values are normalized to untreated control and represent means ± SEM, n = 3–12, *p ≤ 0.05, **p < 0.01, ***p < 0.0001, two-tailed t-test. B) Western blot analysis of NOX5β expression after 2 h incubation with 5 μM hemin with or without 40 μM radicicol. C) Reduced minus oxidized difference spectra of live cells using the Olis Clarity. After addition of cycloheximide to halt protein synthesis, HEK293 cells stably expressing NOXβ grown in heme depleted media with SA were treated with vehicle control (gray), hemin (5 μM, 2 h, red) or hemin and radicicol (5 μM hemin, 40 μM radicicol, 2 h, dashed black). Cells were normalized to live cell number and the UV–vis spectrum was taken before and after addition of sodium dithionite. The oxidized spectrum was then subtracted from the reduced spectrum. Characteristic NOX5 peaks are visible at 429 nm and 558 nm. D, E) Co-immunoprecipitation of Hsp90 with NOX5. HEK293 cells stably expressing NOXβ were treated with 40 μM radicicol and lysed. Equal protein amounts of lysate were incubated with NOX5 antibody and pulled down with Protein A Sepharose beads. After washing the precipitates were subjected to SDS-page and western blot analysis. D) HEK293 cells stably expressing NOXβ were treated with 40 μM radicicol for 0, 0.5, 1 or 2 h. E) HEK293 cells stably expressing NOXβ were treated with vehicle control or 40 μM radicicol and 1 μM ionomycin Ca2+ salt was added just prior to lysing. F) The interaction between Hsp90 and NOX5β in HEK293 cells was probed using the Proximity Ligation Assay (PLA) Duolink kit from Sigma. Red foci represent interaction between the two proteins (<40 nm). Addition of only one antibody does not result in any signal, however, addition of both antibodies results in bright red foci indicating an interaction. HEK293 cells stably expressing NOXβ were treated with vehicle control, 5 μM hemin, 40 μM radicicol, or both hemin and radicicol and interactions between NOX5 and Hsp90 were probed using the Duolink kit. G, H) HEK293 cells stably expressing NOXβ were treated with vehicle control, 5 μM hemin or 40 μM radicicol for 2 h and then lysed. Samples were subjected to SDS-page and western blot analysis in semi-reducing conditions (sample preparation included no reducing agent and heating at 65 °C for 2 min) and probed for NOX5. G) Representative western blots and quantification of NOX5 oligomer/monomer in the G) absence or H) presence of 1 μM ionomycin Ca2+ salt. Values represent mean ± SEM, n = 3. Difference between treatments was assessed using a one-way anova with Tukey’s test with * denoting p < 0.05 and **p < 0.01. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Due to the important role Hsp90 plays in heme insertion into soluble heme proteins [[47], [48], [49], [50], [51]], we hypothesized that Hsp90 may also be facilitating heme insertion into NOX5, and therefore that the suppression of NOX5 activity with radicicol treatment may be due to an inability of NOX5 to bind the provided heme. However, when we obtained difference spectra of cells incubated with heme with and without radicicol treatment we found that Hsp90 inhibition did not suppress the increase in the NOX5 specific heme peak (Fig. 6c), indicating that heme binding was still occurring and that Hsp90 inhibition was blocking NOX5 superoxide production in a different manner.
Hsp90 inhibition by radicicol blocks formation of active NOX5 oligomers. Calcium binding to the EF hands of NOX5 leads to an interaction between the NOX5 N-terminal domain and C-terminal dehydrogenase domain [68,69]. This interaction seems to facilitate formation of a catalytically active NOX5 oligomer mediated through the C-terminal dehydrogenase domain [69,70]. Hsp90 also binds to the C-terminal dehydrogenase domain [66], and treatment with ionomycin to induce NOX5 superoxide production decreases the NOX5:Hsp90 interaction [66]. Based on these reports, we determined the effect of heme and radicicol on NOX5:Hsp90 interactions and NOX5 oligomerization. Co-immunoprecipitation studies found that short (0.5–2 h) treatments with radicicol increased the amount of Hsp90 co-immunoprecipitated with NOX5 (Fig. 6d, Supp. Fig. 5a and b). More importantly, a 2 h treatment with radicicol blocked the dissociation of Hsp90 from NOX5 upon treatment with ionomycin Ca2+ salt (Fig. 6e, Supp. Fig. 5c and d). To further investigate these results we used the proximity ligation assay Duolink, which results in red foci when two target proteins (NOX5 and Hsp90) are in close proximity (<40 nm). Adding either single antibody alone resulted in no visible foci (Fig. 6f), while addition of both antibodies (NOX5 and Hsp90) resulted in bright red foci indicating the proteins were in close proximity. We confirmed that NOX5 and Hsp90 interact in resting cells (Fig. 6f) and that this interaction is increased in the presence of radicicol (increases red foci), even with co-incubation with heme (Fig. 6f).
We then treated NOX5 expressing cells with heme (5 μM hemin, 2 h) or radicicol (40 μM, 2 h), and assessed the effects of these treatments on NOX5 oligomerization with and without ionomycin Ca2+ salt by performing SDS-page and western blot analysis on normalized lysates in semi-reducing conditions (no reducing agent, heating to 65 °C). Under these conditions NOX5 exists as a mixture of monomers and oligomers (Fig. 6g and h). Heme treatment appears to increase NOX5 oligomerization in the absence of ionomycin Ca2+ salt (Fig. 6g), although this increase was not statistically significant. Importantly, radicicol decreased NOX5 oligomerization specifically in response to ionomycin Ca2+ salt (Fig. 6h). Taken with the co-immunoprecipitation and proximity ligation assay results, this indicates that radicicol may inhibit Hsp90 dissociation from NOX5 even after Ca2+ binding to the NOX5 EF-hand domains. These changes in NOX5:Hsp90 binding and NOX5 oligomerization correlate well with changes in NOX5 activity (Fig. 6a), and suggest that heme and Hsp90 dynamically regulate NOX5 activity by controlling its oligomeric state (Fig. 7).

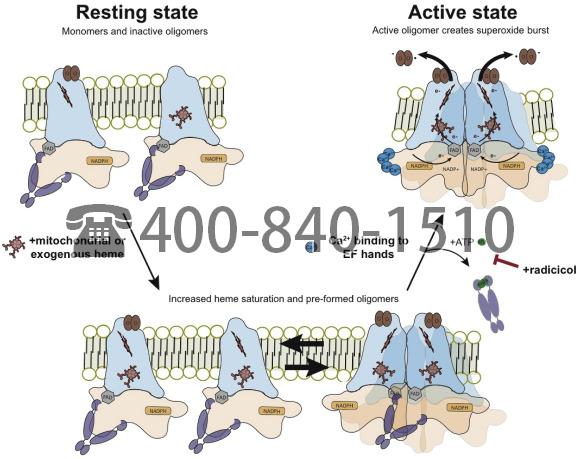
Fig. 7. Model showing the influences of heme and radicicol on NOX5 oligomerization and activity. Under normal resting conditions NOX5 exists as apo- or semi-heme bound monomers and inactive but heme saturated oligomers. Upon calcium binding by N-terminal EF hands, an N-terminal:C-terminal dehydrogenase domain interaction [68] facilitates NOX5 oligomer formation and activity. The heme saturated oligomers undergo conformational rearrangements to form active oligomers and produce a superoxide burst. Increases in cellular heme levels lead to a build-up of heme saturated monomers and preformed oligomers. The pre-existence of these species leads to an increase in superoxide production upon stimulation with ionomycin. Radicicol treatment stabilizes the interaction between Hsp90 and NOX5 and blocks NOX5 oligomerization and activity upon ionomycin stimulation.
Heme and Hsp90 dynamically regulate NOX5 activity through controlling oligomeric state and conformation. Our findings suggest that NOX5 exists in a semi-heme bound state and that calcium binding induces formation of catalytically-active NOX5 oligomers (Fig. 7). Previous work has shown that co-transfection of inactive NOX5 variants has a dominant negative effect on NOX5, decreasing overall activity [70]. We hypothesized that if the oligomer is the catalytically active form of NOX5, it may be possible for electron transfer to occur from the dehydrogenase domain of one monomer to the heme binding transmembrane domain of a neighboring monomer. To test this, we made NOX5 variants with deactivating substitutions in the C-terminal dehydrogenase domain [65,70] (Pro420Ala, Pro549His, and Asp638Ala) and tested their effect on superoxide production when co-transfected with empty vector, WT NOX5β and the heme binding deficient variant His282Ala (Supp. Fig. 6). All variants displayed either little (Pro420Ala) or no (Pro549His, Asp638Ala and His282Ala) superoxide production when co-transfected with empty vector. The variants had little effect on NOX5 superoxide production when co-transfected with WT NOX5β, but all four variants dramatically decreased the response to heme (Supp. Fig. 6). This further supports a model in which heme increases superoxide production by NOX5 by affecting the conformation of its oligomeric state. Finally, co-transfection of C-terminal dehydrogenase domain variants (Pro420Ala, Pro549His, and Asp638Ala) with the heme binding deficient variant His282Ala showed no change from the variants transfected with empty vector (Supp. Fig. 6). The lack of rescue indicates that each monomer within an oligomer must be fully capable of its own electron transfer, and that rather than facilitating electrons transferring in trans, the oligomer formation likely facilitates the electron transfer in cis, possibly by affecting the conformational state and rigidity of NOX5 subunits. The blunting of the heme effect with co-transfection of inactive variants supports a role for changes in heme saturation influencing the formation of NOX5 oligomers that are capable of transitioning to the active state.
4. Conclusions
Little is known about the maturation and regulation processes of integral membrane heme proteins like NOX enzymes. Here we show that the transmembrane signaling enzyme NOX5 is not heme-saturated in mammalian cells, and that cell heme levels along with Hsp90 binding coordinate to dynamically regulate the oligomerization state and activity of NOX5. This new layer of regulation further integrates the activity level of NOX5 into the global state of the cell, and can combine with other regulatory mechanisms to fine-tune NOX5 responses to stimuli. Our current findings appear to explain the NOX-dependent response to heme in alveolar macrophages [45] and epithelial cells [46] and may signify that a broader level of regulation is also possible for the NOX enzyme family and other transmembrane heme proteins. Whether other NOX family members are similarly regulated by cell heme levels remains to be definitively determined, however, given the highly conserved nature of their heme binding sites, this remains a possibility.
A model of NOX5 regulation that is consistent with results to date is depicted in Fig. 7. In the resting state NOX5 exists in cells as a mixture of apo- and semi-heme saturated monomers along with some heme-replete oligomers. All are associated with Hsp90. Hsp90 binding to the C-terminal domain of NOX5 appears to play dual roles; 1) it stabilizes the dehydrogenase domain and 2) antagonizes the formation of active NOX5 oligomers. An increase in intracellular heme allows heme to fill the unoccupied binding sites in NOX5. This promotes formation of inactive but fully heme-bound NOX5 oligomers that remain associated with Hsp90. This larger pool of NOX5 oligomers can then be activated when cell calcium flux is triggered with ionomycin. Calcium binding to the EF hand domains causes conformational changes which appear to displace Hsp90 and relieve it’s auto-inhibitory interaction [68,69]. The Hsp90 inhibitor radicicol, by inhibiting Hsp90 ATP hydrolysis and conformational cycling [71,72], appears to stabilize the NOX5:Hsp90 interaction and block Hsp90 displacement upon calcium binding, thus blocking formation of catalytically-active NOX5 oligomers. These findings lead to further questions about the exact conformation of these inactive vs. active oligomers and whether we can identify additional protein and small molecule regulators. Disruption or stabilization of different conformational states can also provide novel druggable targets.
Transmembrane heme proteins are an especially interesting case for oligomerization control because their degrees of freedom are already constrained by their location in the membrane. The presence of lipid rafts and proteins that regulate activity through physical interactions, such as Caveolin-1 (Cav-1) [44], make transmembrane heme proteins well-suited for dynamic regulation through control of their oligomeric state. Indeed, Cav-1 has been shown to inhibit NOX5 activity [44]. It is tempting to speculate it may act through a mechanism similar to what we propose for Hsp90, where its interaction with NOX5 antagonizes oligomerization and also prevents inactive oligomers from switching to the active conformation. Furthermore, Hsp70 and CHIP have also been shown to play a role in NOX5 regulation [67], and a full understanding of how these and other chaperones coordinate in regulating NOX5 expression and activity in response to stimuli will greatly enhance our understanding of the mechanisms controlling NOX5 signaling networks.
Prior to our study, the ability of heme or chaperones to mediate changes in oligomerization state have only been reported for a few soluble heme proteins including iNOS [73] and sGC [48]. In these proteins, heme binding is required for their homodimer or heterodimer formation, respectively, and then the subsequent binding of additional cofactors (iNOS [73]) or NO (sGC [48]) creates the active oligomers. Our findings indicate that NOX5 similarly requires heme binding for oligomer formation, which poises the inactive oligomers for activation by Ca2+ binding and release of Hsp90. For sGC Hsp90 is crucial for heme insertion [47,49], and similar to NOX5, Hsp90 dissociation is required for the formation of active oligomers [47,49]. There remains the possibility that Hsp90 is involved in heme insertion into NOX5, although this role would have to be ATPase independent. The existence of layered regulatory mechanisms to control the activities of these heme dependent enzymes may be more widespread than previously understood, and would accordingly allow regulation of these proteins to respond to changes in an array of cellular factors. Future studies specifically focused on heme delivery, insertion and removal from NOX5 and other NOX enzymes will further inform our understanding of these crucial processes. A promising target for involvement in NOX5 heme delivery and insertion is GAPDH, a heme chaperone required for heme delivery to iNOS [64,74] and sGC [75]. Understanding how these chaperones work together to control NOX heme content and activity will further our understanding of how these processes are integrated with overall cellular responses to environmental changes.
Regulation of the NOX5 oligomeric state and activity by heme and Hsp90 has biomedical impact in systems where NOX5 has been implicated to play a role, including immunity [36] and cardiovascular health [35] and in cancers [25,26], diabetes [14,27], and cardiovascular disorders [[28], [29], [30], [31], [32], [33], [34]]. The dynamic effects of heme and Hsp90 on NOX5 activity represent a new regulatory mechanism that could respond in cases of anemia, hemorrhage or even during transient changes in intracellular heme levels, such as those identified in failing hearts [76]. Overall these findings illuminate a novel form of NOX5 regulation with downstream impacts on the redox state of the cell and modulation of signaling pathways. Having now established that these heme dependent effects are relevant in cells expressing endogenous NOX5, such as BET1A epithelial cells, we can now assess how changes in NOX5 activity affect cellular responses and phenotypes. More broadly, this work has provided the framework to investigate more closely how cell heme levels and Hsp90 regulation of NOX5 impact its functions in physiology and pathology.
Declaration of competing interest
The authors declare no conflicts of interest.
Acknowledgments
We thank S. Comhair and L. Mavrakis for the BET1A cells and technical assistance, and A. Ghosh for meaningful discussions. This work was supported by NIH grants P01 HL081064, P01 HL103453 (D.J.S), and K99 HL144921 (E.A.S.), and an American Heart Association Postdoctoral Fellowship (E.A.S.)